Фармакологические свойства меланина
Pharmacological Properties of Melanin and its Function in Health
Adila Salih ElObeid1 , Afaf Kamal-Eldin2 , Mohamed Anwar K. Abdelhalim3 and Adil M. Haseeb3 1 King Abdullah International Medical Research Centre, National Guard & Health Affairs, Riyadh, Saudi Arabia, 2 Department of Food Science, United Arab Emirates University, Allain, United Arab Emirates and 3 Physics and Astronomy Department, King Saud University, Riyadh, Saudi Arabia
(Received 28 September 2016; Accepted 14 December 2016)
Abstract: The biological pigment melanin is present in most of the biological systems. It manifests a host of biological and pharmacological properties. Its role as a molecule with special properties and functions affecting general health, including photoprotective and immunological action, are well recognized. Its antioxidant, anti-inflammatory, immunomodulatory, radioprotective, hepatic, gastrointestinal and hypoglycemic benefits have only recently been recognized and studied. It is also associated with certain disorders of the nervous system. In this Minireview, we consider the steadily increasing literature on the bioavailability and functional activity of melanin. Published literature shows that melanin may play a number of possible pharmacological effects such as protective, stimulatory, diagnostic and curative roles in human health. In this Minireview, possible health roles and pharmacological effects are considered.
Melanin is a ubiquitous biological pigment, which is present in mammalian skin, hair, eyes, ears and the nervous system. It is known to exist in birds' feathers, squid's ink, insects, plants and many other biological systems 1. Recently, it has received considerable attention and research. Melanin is abundant in many human diets, but no research has been attempted to evaluate effects of daily intake. This Minireview covers possible pharmacological and health effects of melanin.
Melanin is classified into three groups: eumelanin's, pheomelanin's and allomelanins. Melanins of the nervous system are known as neuromelanins. Colours of the eumelanins, which are most commonly found in animals, are black or brown. They are highly insoluble pigments that form within specialized cells known as melanocytes. Enzymatic action of the enzyme tyrosinase on the amino acid tyrosine produces melanin. In their primary biosynthetic pathway, tyrosine is hydroxylated to form the catecholamine 3,4-dihydroxyphenylalanine (DOPA), which is then oxidized to form 3,4-dioxyphenylalanine (dopaquinone) before cyclization to 5,6-indole quinones and their subsequent polymerization to form melanin. Similar to the biosynthesis of eumelanin, melanin known as pheomelanin is biologically synthesized, except that a precursor containing Sulphur is incorporated in the structure 1. Eumelanin is usually observed as brownish to dark black color of the skin and hair, while pheomelanins are reddish and yellowish in color. Many biological systems produce a combination of the two types of melanin. Red-haired people usually have more of pheomelanin in their hairs and skins. Many studies have shown that people with pheomelanin as the predominant pigment are susceptible to more photodamage than people with predominant eumelanin in their skins. The importance of melanin as a vital biological molecule is well recognized. It has even been suggested that melanin has played an evolutionary role as a central ‘organizing molecule', assuming functions similar to those of enzymes in present evolutionary systems 2. Despite this recognition, many of the Minireview basic functions of melanin remain poorly understood.
Health Effects of Melanin
Sunscreening and radioprotective effects of melanin
Melanin from natural sources has reported to possess a broad spectrum of biological activities, which include protection against UV radiation, enzymatic lysis, damage by oxidants, resistance to drugs by pathogens, protection of insects against bacteria and antiviral protection 3-6. Additionally, melanin has been shown to chelate metal ions and to act as a physiological redox buffer 7, 8. The use of melanin in cosmetics and sunscreens has been adopted by many manufacturers in an attempt to mimic the natural role of these molecules in the skin. The protective effect of sunscreen is rated using the Sun Protection Factor (SPF) scale, and it is thought that a higher SPF value indicates a better protective capacity. In 2011, Huang et al. 9 demonstrated that the SPF value of gel formulations increased with the addition of melanin extracts from the berry of Cinnamomum burmannii and Osmanthus fragrans. Revskaya et al. (2012) 10 investigated the possibility of creating an efficient oral radioprotectant based on melanin using edible black mushrooms prior to administration of 9 Gy total-body irradiation in mice. The location of the mushroom-derived melanin in the body before irradiation was determined by in vivo fluorescent imaging. Ingestion of black mushrooms protected 80% of mice from the lethal dose, whereas control mice, or those given mushrooms lacking melanin, died from a gastrointestinal syndrome. Notably, mice that were given white mushrooms supplemented with melanin survived at the same rate as mice that were given black mushrooms. The authors concluded that melanin-containing mushrooms can provide significant protection against radiation and could be developed into oral radioprotectants. Similarly, Kunwar A et al. (2012) 11 studied the probable mechanism of the radioprotective action of extracellular melanin (isolated from the fungus Gliocephalotrichum) in mice after exposure to 7 Gy whole-body irradiation. They found that administration of melanin at an effective dose of 50 mg/kg of body-weight increased the survival of mice by 100%. Mice that received melanin displayed reversion of radiation-induced reduction in extracellular signal-regulated kinase phosphorylation in splenic tissue. The authors suggest that this mechanism is a key feature that mediates melanin's radioprotective effects. In some diseases, the excess free radicals that are created by UV radiation in the skin are suggested to be involved in UV-induced photocarcinogenesis. Valavanidis et al. 12 used electron spin resonance (ESR) to carry out in vivo studies of free radicals' mechanisms in UV-induced skin photocarcinogenesis.
Interaction of melanin with drugs and metals and its related pharmacological properties
In 1993, Larsson conducted extensive pharmacological and physiochemical studies on the interaction of melanin with metals and drugs 6. This work showed that various drugs and other chemicals, such as organic amines, metals and polycyclic aromatic hydrocarbons, readily bind to melanin and are retained in pigmented tissues for long periods. The physiological significance of this metallic-binding property of melanin is not clear, but one suggestion is that melanin protects pigmented cells and adjacent tissues by adsorbing potentially harmful substances, which are then slowly released in non-toxic concentrations. However, Larsson also mentioned that long-term chemical exposure may lead to build-up of high levels of noxious chemicals in melanin, which may ultimately cause cellular degeneration and secondary lesions in surrounding tissue 6. Melanins may thus act as scavengers of toxic material in foods and may contribute to the regulation of some metals (such as iron and copper) that are of importance in metabolic and physiological processes. More recently, Karlsson and Lindquist 13, 14 have reviewed the toxicological implications of melanin's and neuromelanin's binding of drugs and chemicals. Many studies suggest that specific retention of drugs and metals by melanin protects the cells initially but also serves as a depot that slowly releases accumulated compounds and may cause toxicity on overexposure. Compounds with high neuromelanin affinity have been implicated in the development of adverse drug reactions in the central nervous system (CNS) as well as in the aetiology of Parkinson's disease (PD).
In addition, melanin could also interact with orally administered drugs and be used as a vehicle for drug delivery. For example, Lei et al. (2008) 15 conducted an interesting study on the use of a melanin–iron complex to induce remission of iron-deficiency anaemia. Treatment with the melanin complex led to higher bioavailability of iron and fewer side effects than did treatment with standard drugs. The melanin–iron complex significantly reduced symptoms, suggesting that melanin–iron complexes might help improve haematopoietic function and could be exploited as a safe, efficient new iron tonic. Seniuk et al. (2011) 16 reported various favourable effects of melanin complexes on pure cultures of Helicobacter pylori, Candida albicans, Herpes vulgaris I and HIV-1, both in in vitro and in vivo animal models. The authors concluded that these melanin complexes could be used as a source of biopolymers for the creation of new agents with wide applications in infectious pathology.
Antioxidant effects of melanin
Melanins from various sources exhibit significant antioxidant activity 8, 17, 18. The role of melanin as a scavenging or quenching molecule on superoxide anions, and singlet oxygen species has been discussed by Tada M et al. (2010) 19 who used ESR and spectrophotometric methods to show that melanin potently interacts with reactive oxygen species that are generated in certain physiological reactions.
These antioxidant melanins include extracts from the berry of Cinnamomum burmannii and Osmanthus fragrans, which also have concentration-dependent metal-chelating activities. Hoogduijn et al. (2003) 20 observed that melanin protects melanocytes and keratinocytes from DNA damage caused by hydrogen peroxide, indicating that the pigment has an important antioxidant role in the skin.
Melanin extracted from tea leaves was found to inhibit the oxidation of low-density lipoproteins, which supports the idea of an inhibitory effect of melanin against peroxyl radicals 21. It has also been demonstrated that a reduced form of tea melanin behaves as a free radical chain-breaking antioxidant and competes with β-carotene as a substrate for chain-propagating peroxyl radicals. In addition, reduced tea melanins were found to be more effective as anticarcinogenic agents than non-reduced melanins.
The physicochemical characterization and antioxidant activity of melanin from a strain of Aspergillus bridgeri have been studied by Kumar et al. (2011) 22, who demonstrated that melanin exhibits significant free radical scavenging activity. This work suggests that melanin may have potential applications as a natural antioxidant in the cosmetic and pharmaceutical industries.
Simon et al. 23 have suggested that complex melanins, built of eumelanin and pheomelanin fractions, exhibit an antioxidant effect due to the action of eumelanins, while pheomelanins tend to cause a pro-oxidant effect. According to this, the antioxidant behaviour of melanin should be considered as due to a combination of two opposite effects. But as eumelanin is the predominant fraction in most of the biological organs, what is observed is mostly an antioxidant effect pertaining to eumelanin.
Enhancement and modulation of the immune system by melanin
A number of previous studies have shown that both plant- and synthetic melanin can modulate cytokine production and enhance several immune parameters 24-27.
Recently, it has been demonstrated that animal and fungus melanin (derived from rat and Aspergillus fumigatus, respectively) also modulates cytokine production 28, 29. Sava et al. (2001) 21 extracted melanin-like pigments (MLPs) from black tea and showed that oral administration of MLPs to mice significantly stimulated splenic lymphoid tissue. Later, Hung et al. demonstrated that melanin extracted from different tea species induced cytokine production, with green tea melanin being at least 100 times more active than black tea melanin 30. They have also reported that antibody-secreting cells produced significantly more antibodies in animals treated with tea melanin (32–34%) than did antigen controls. Similarly, Al-Mufarrej et al. (2006) 31 showed that, in albino rats, black seed melanin induced a high and long-lasting antibody response to sheep red blood cells by stimulating the immune system. The immunostimulatory effects of melanin preparations from 30 traditional medical herbs were studied and patented by Pasco et al. (2005) 32. The patent authors reported that the melanins with the highest levels of activity were found in Allium sativum, Tabebuia spp., Serenoa repens and Echinacea spp.
Pugh et al. (2005) 24 demonstrated that ingestion of these melanins by mice caused dendritic cells in Peyer's patches to secrete high levels of IgA and interleukin-1 (IL-1). In these studies, spleen cells from mice that were fed melanins exhibited increased production of interferon gamma (IFG-ɣ) as a result of a shift in the balance of T helper 1 and T helper 2 cells (Th1 and Th2, respectively) in favour of Th1. Avramidis et al. (1998) 33 found that grape melanin modulates the production of IL-1, IL-6 (interleukin-6) and tumour necrosis factor-α (TNF-α) and significantly inhibited adjuvant-induced disease in rats. They suggested a possible role for melanin in inhibiting lymphocyte Th1 (T4 or T8), which led to the suppression of adjuvant-induced disease development. Recently, the important role of Toll-like receptors (TLRs) and their corresponding ligands in modulating cytokine production has been reported 34. TLRs are transmembrane or cytoplasmic receptors that recognize conserved molecular patterns of bacteria, fungi and viruses. They are members of the IL-1 receptor superfamily and share significant homology in their cytoplasmic regions 35, 36.
The most well-defined exogenous ligands for TLRs are lipopolysaccharide (LPS) for TLR4 and TLR2 and peptidoglycans and lipoproteins for TLR2 37, 38. In addition to exogenous ligands, TLR4 and TLR2 can be stimulated by various endogenous ligands such as heat-shock proteins (HSPs) and fibronectin 39-41. Plant melanins represent a new class of polymers that are recognized by the TLR family. Melanin isolated from Echinacea and Nigella sativa seeds were shown to activate monocytes by binding TLR-2 and TLR-4, respectively 42. Ligand binding to TLRs induces dimerization and recruitment of various adaptor molecules, which induce the production of various cytokines and activate downstream signalling pathways such as the myeloid differentiation factor 88 (MyD88) pathways. These cascades primarily include the nuclear factor-kB (NF-kB), MAP-kinase and interferon regulatory factor-3 and interferon regulatory factor-7 (IRF-3 and IRF-7) signalling pathways. In addition, TLR4 activation can induce a MyD88-independent pathway, TRIF, and activate the IRF-3 pathway 43 (fig. 1).
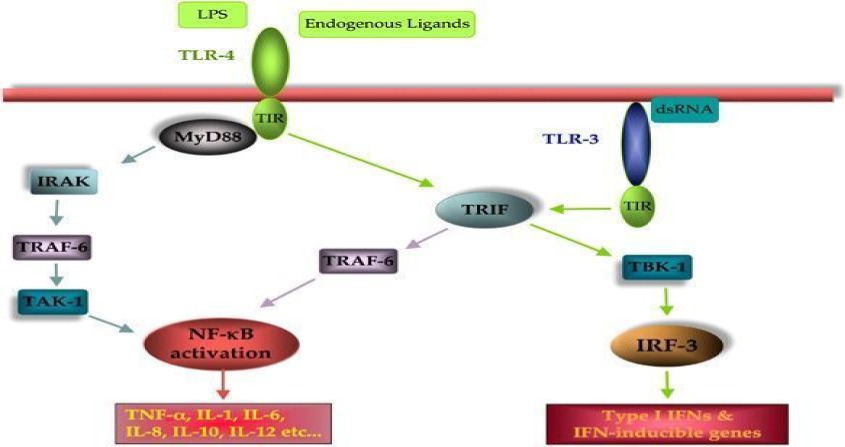
TLR signalling pathways. Transmembrane TLRs (represented here by TLR4) recognize external ligands (exogenous and endogenous), whereas cytoplasmic TLRs (TLR3) recognize intracellular signals. When activated, the majority of TLRs induce activation of NFκ-B (early-phase activation) and cytokine production in a MyD88-dependent manner. However, TLR4, like TLR3, can also signal in a MyD88-independent manner and induce expression of type I interferon (IFN) and IFN-inducible proteins in addition to late-phase NFκ-B activation 43.
Melanin extracted from Nigella sativa L. was shown to directly activate TLR-mediated signalling pathway in monocytes, peripheral blood mononuclear cells and in the THP-1 human monocytic cell line 44. Recently, Muller et al. (2013) 28 demonstrated that melanin extracted from murine melanoma B16F1 cells increased secretion of chemokines MIP-1β (CCL4) in dendritic cells derived from primary monocytes and human monocytic cell line (MoDCs and THP-1, respectively). Kunwar A. et al. (2012) 11 reported that melanin isolated from the fungus Gliocephalotrichum simplex reduced oxidative stress in hepatic tissue and abrogated immune imbalance by reducing the production of cytokines (IL6 and TNF-α) in BALB/C mice.
Most botanicals with immune-enhancing properties contain high molecular weight LPSs or lipoproteins. Consequently, much of their in vitro macrophage-activating properties may be a result of contamination with these agents 22. However, subjecting botanical extracts to high alkalinity or high acidity destroys the LPSs 45-48 and causes subsequent loss of their biological activities, including cytokine modulation 49, 50. A number of studies have shown that melanin treated extensively with acids (pH 2) and alkali solutions (pH 14) still maintains their immunogenic properties 51. Conversely, it is well known that melanin has high affinities for various molecules that may bind to them 7, and covalently bonded polysaccharides have been detected in melanin that survived enzymatic and acid treatments.
A possible problem that may arise in the use of melanins as pharmacological agents for modulation of the immune system is an adverse effect of excessive immune system activation. It has been reported that extracellular neuromelanin can activate the CNS microglia and may ultimately induce neurodegeneration via inflammatory pathways 52.
Modulation of gastrointestinal health of melanin
Previous studies on the effect of animal melanin on gastric ulcers revealed that melanin extracted from marine squid and Ommastrephes bartrami Lesuel inhibited both phenylbutazone-induced ulceration in gastric mucosa and secretion of gastric juice in rats 53-59. Similar benefits on gastric health were obtained using an aqueous suspension of ground Nigella sativa seeds that contained melanin. In addition, melanin can prevent formation of gastric ulcers induced by necrotizing agents such as indomethacin. Moreover, melanin can drive replenishment of the levels of mucus in ethanol-depleted gastric cell walls 57. Recently, it was shown that pure melanin extracted from Nigella sativa seeds strongly protects against ulcers induced by alcohol, indomethacin, stress or the combined ulcerogenic action of both stress and aspirin 58, 59.
The lamina propria, situated just below the epithelium, consists of highly vascularized loose connective tissue with an abundant supply of lymphatic nodules, lymphocytes, plasma cells and macrophages. It provides the first line of immunological defence against invasion by bacteria, viruses and harmful macromolecules. The expression of different TLR receptors 1-9 in this layer plays a major role in the protective process 59, 60. TLRs are constantly exposed to and activated by microbial ligands produced by pathogenic and commensal bacteria 61, 62. Under normal steady-state conditions, TLRs maintain homeostasis and epithelial functions 63-65, but they are up-regulated during intestinal inflammation and inflammatory bowel disease 66-68. The expression of TLR2 and TLR4 mRNA and protein is detectable in the epithelial cells of the stomach, small and large intestines 69, 70. Activation of TLR2 and TLR4 receptors by dietary plant melanins and the similarity between melanin and LPS in TLR4 activation have already been reported.
Rakoff-Nahoum et al. 63 showed that the TLR4 and TLR2 ligands, LPS and lipoteichoic acid from commensal bacteria, can protect against intestinal epithelial damage, severe bleeding and mortality induced by dextran sodium sulphate (DSS). They also showed that LPS up-regulates the expression of heat-shock proteins (HSPs), HSP25 and HSP72, which play a cytoprotective role in intestinal epithelial cells 71. It is thought that LPS protects the gut by inducing cyclooxygenase (COX)-2 and prostaglandin (PG) E2 72. Recently, another TLR4 ligand, hyaluronic acid (HA), was implicated in the protection against DSS-induced colitis 73, 74. Intraperitoneal, endogenous and exogenous administration of HA activated TLR4-induced COX-2 and PGE2 (fig. 2). The similarity between melanin and TLR4 ligands suggests that melanin could have a similar protective action in the gastrointestinal tract.
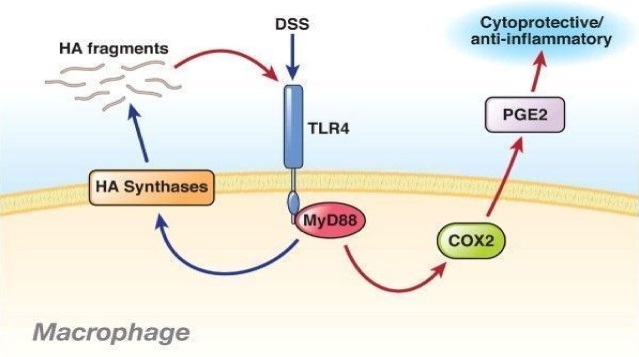 DSS induces HA fragments that function through TLR-4 to induce anti-inflammatory effects in colitis. DSS administration is a commonly used model of colitis. Zheng et al. have provided complex insights into the effect of DSS in the gut and demonstrated that DSS can act through TLR-4 in macrophages to induce HAS, which leads to the production of HA fragments (blue lines). In turn, these fragments also act through TLR-4, induce COX-2 and lead to PGE2 production, which has a cytoprotective effect and is anti-inflammatory in the epithelium (red lines). These fragments can also induce additional fragment production in a feed-forward effect, propagating the anti-inflammatory response. Administration of HA fragments is anti-inflammatory in the DSS model, possibly indicating a new therapeutic approach for colitis 74.
DSS induces HA fragments that function through TLR-4 to induce anti-inflammatory effects in colitis. DSS administration is a commonly used model of colitis. Zheng et al. have provided complex insights into the effect of DSS in the gut and demonstrated that DSS can act through TLR-4 in macrophages to induce HAS, which leads to the production of HA fragments (blue lines). In turn, these fragments also act through TLR-4, induce COX-2 and lead to PGE2 production, which has a cytoprotective effect and is anti-inflammatory in the epithelium (red lines). These fragments can also induce additional fragment production in a feed-forward effect, propagating the anti-inflammatory response. Administration of HA fragments is anti-inflammatory in the DSS model, possibly indicating a new therapeutic approach for colitis 74.
Hepatoprotective effects of melanin
Melanin extracted from different species of tea displays protective effects against hydrazine-induced liver injury. Sava et al. (2003) 75 showed that administration of tea-derived melanin-like pigments (MLPs) to rats 30 min. before administration of hydrazine prevented both the development of heavy liver intoxication and the rise of liver serum alanine transferase (ALT) activity. Furthermore, treatment was associated with lower malondialdehyde (MDA) concentrations and decreased glutathione levels in the liver 75. In another study, they reported that tea melanin, in addition to the aforementioned effects, prevents the production of free radicals and the formation of 8-hydroxy-deoxyguanosine (8-OH-dG) DNA adducts 76. Later, the same group studied the effects of reduced and non-reduced tea melanin (RTM and NRTM, respectively) against 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD)-induced toxicity in mice 77. TCDD enhances oxidative stress by mediating hydrogen peroxide production, inducing cytochrome P450, family 1, subfamily A, polypeptide 1 (CYP1A1) and increasing 8-OH-dG production 78.
The authors concluded that both RTM and NRTM suppress expression of the CYP1A1 gene, which prevents the activation of cytochrome P450 isozyme in the livers of exposed mice by suppressing aryl hydrocarbon receptors (AhRs) 77. Similar results were obtained with Camellia sinensis tea melanin (CSTM) 26. CSTM inhibited the TCDD-induced transformation of AhRs by suppressing downstream AhR activation of the genes associated with TCDD toxicity. Additionally, Avramidis et al. showed that grape melanin strongly inhibits in vitro lipid peroxidation of rat liver microsomal membranes and protects in vivo hepatic peroxidation that occurs in adjuvant-induced disease rats 33. Recently, ElObeid et al. (2015) 79 have shown that Nigella sativa L. melanin has prevented the CCl4-induced liver injury in Wistar rats. Pre-treatment of the rats with melanin resulted in significant reduction in the CCl4-induced levels of aspartate aminotransferase (AST), ALT and MDA. The authors proposed the observed effect to IL-6 production via TLR4 activation by melanin.
Anticarcinogenic effects of melanin
In 1997, Kamei et al. showed that protein-free allomelanins extracted from black soya beans and black sesame seeds, suppressed the growth of HCT-15 cells (a cultured human intestinal carcinoma cell line) and Meth/A cells (generated from a Balb/C mouse lymphoma) 80. Subsequent work by the same group using flow cytometry studies showed that the addition of a high concentration of protein-free allomelanins (400 mg/ml) to HCT-15 culture medium blocked the S phase of the cell cycle 81. A study on the in vivo effect of these melanins in Balb/C mice inoculated intraperitoneally with Meth/A cells showed that the allomelanins significantly increased survival in the experimental group relative to controls 80. Offen et al. demonstrated that synthetic DOPA-melanin caused 50% cell death in the PC-12 cell line. On the basis of this work, they suggested a possible role of melanin in inducing apoptosis in PC12 cells 82. Blinova et al. studied the effect of 19 melanin preparations isolated from black yeast fungi on keratinocyte and fibroblast proliferation. They concluded that melanin modulates the proliferation of both cell types with a more pronounced effect on keratinocytes 83.
Anti-inflammatory effects of melanin
A remarkable anti-inflammatory effect of melanin has been reported by Mimura et al. 84. This group tested the melanin extracted from Ommastrephes bartrami LESUEL on carrageenan-induced rat oedema. They reported that administration of melanin, by either intravenous or intraperitoneal injection, effectively suppressed the induced subacute and acute inflammation. Similarly, Avramidis et al. (1998) 30 studied the effect of grape melanin on carrageenan-induced oedema, as well as on oedemas produced by other phlogistics. They reported that melanin interferes with the prostaglandin, leukotriene and/or other complement systems that mediate inflammation. They also demonstrated a strong inhibitory effect of grape melanin on suppressing primary inflammation in adjuvant-induced disease in rats 30. Recently, Nilima et al. studied the role of melanin pigmentation in gingival inflammation in pigmented and non-pigmented groups 85. Compared with the non-pigmented group, the pigmented group showed lower numerical values for clinical markers of inflammation, such as gingival index and bleeding index, which suggests a protective activity of melanin against gingival inflammation. Finally, El-Obeid et al. (2016) have studied the anti-inflammatory effect of Nigella sativa L melanin against formalin-induced rat-paw oedema. Their results have shown that topical application of melanin has a strong anti-inflammatory action and that melanin is more effective than hydrocortisone 86.
Conclusion
Research has revealed many interesting pharmacological effects of melanin. However, the accepted practical approach to describe melanin is through its heterogeneous nature and its known variety of functions. Using specific and definitive research, only some advances into the antioxidant, anti-ulcer, anti-inflammatory, hepatoprotective, antidiabetic, hypoglycaemic and immunostimulating effects of melanin has been obtained. Future work on the pharmacological properties of melanin in relationship to its generally accepted health effects is needed. How melanin exerts its functions in situ in living creatures is poorly understood; only the obvious differences that result from its presence or absence in human tissue have been described in detail. Ingestible melanin, as a component of many foods, may play certain roles in human health. Although most of these effects are considered beneficial, many are not yet well understood and some of them may lead to some adverse effects on the human body. Extensive efforts to unravel the complex problems of how it may interfere with disease processes or how it could function as a drug through gastrointerstitial or intravenous routes have yet to be undertaken.
Acknowledgements
The authors would like to extend their sincere appreciation to the Deanship of Scientific Research at King Saud University for its funding of this research through the Research Group Project No. RGP-285.
References
- 1. Melanins. Hermann Press, Paris, France, 1968.
- 2. Melanin: the organizing molecule. Med Hypotheses 1983; 11: 1– 139.
- 3, , , , . Biosynthesis of fungal melanins and their importance for human pathogenic fungi. Fungal Genet Biol 2003; 38: 143– 58.
- 4, , . Melanin and virulence in Cryptococcus neoformans. Curr Opin Microbiol 2000; 3: 354– 8.
- 5. Pathogenic roles for fungal melanins. Clin Microbiol Rev 2000; 13: 708– 17.
- 6, . Selective antiviral activity of synthetic soluble L-tyrosine and L-dopa melanins against human immunodeficiency virus in vitro. Antiviral Res 1991; 15: 11– 25.
- 7. Interaction between chemicals and melanin pigment. Cell Res 1993; 6: 127– 33.
- 8, , , . Interactions of melanin with metal ions. Electron spin resonance evidence for chelate complexes of metal ions with free radicals. J Am Chem Soc 1978; 100: 3922– 6.
- 9, , , , , et al. Antioxidant activities and UV-protective properties of melanin from the berry of Cinnamomum burmannii and Osmanthus fragrans. Med Chem Res 2011; 20: 475– 81.
- 10, , , , , et al. Compton scattering by internal shields based on melanin-containing mushrooms provides protection of gastrointestinal tract from ionizing radiation. Cancer Biother Radiopharm 2012; 27: 370– 576.
- 11, , , , , et al. Melanin: a promising radio-protector: mechanisms of actions in a mice model. Toxicol Appl Pharmacol 2012; 264: 202– 11.
- 12, , , , . Studies in vivo by electron spin resonance of free radical mechanisms implicated in UV-induced skin photocarcinogenesis. Int J Cosmet Sci 1995; 17: 157– 63.
- 13, . Melanin affinity and its possible role in neurodegeneration. J Neural Transm 2013; 120: 1623– 30.
- 14, . Melanin and neuromelanin binding of drugs and chemicals: toxicological implications. Arch Toxicol 2016 Aug; 90: 1883– 91.
- 15, , , , , . Effect of squid ink melanin-Fe on iron deficiency anemia remission. J Food Sci 2008; 73: H207– 11.
- 16, , , , , et al. Anti-infective properties of the melanin-glucan complex obtained from medicinal tinder bracket mushroom, Fomes fomentarius (L.: Fr.) Fr(Aphyllophoromycetideae). Int J Med Mushrooms 2011; 13: 7– 18.
- 17, , , , . Antioxidant properties of fungal melanin pigments. Appl Biochem Microbiol 2000; 36: 491– 5.
- 18, , , . Identification and antioxidant activity of melanin isolated from Hypoxylon archeri, a companion fungus of Tremella fuciformi. J Basic Microbiol 2008; 48: 217– 21.
- 19, , . Scavenging or quenching effect of melanin on superoxide anion and singlet oxygen. J Clin Biochem Nutr 2010; 46: 224– 8.
- 20, , , , . Melanin protects against H2O2- induced DNA strand breaks through its ability to bind Ca2+. Exp Cell Res 2004; 294: 60– 7.
- 21, , , , . Isolation and characterization of melanic pigments derived from tea and tea polyphenols. Food Chem 2001; 73: 177– 84.
- 22, , , , . Physicochemical characterization and antioxidant activity of melanin from a novel strain of Aspergillus bridgeri. ICTF-Lett Appl Microbiol 2011; 53: 350– 8.
- 23, , , . Current challenges in understanding melanogenesis: bridging chemistry, biological control, morphology and function. Pigment Cell Melanoma Res 2009; 22: 563– 79.
- 24, , , , , et al. Melanin: dietary mucosal immune modulator from Echinacea and Other botanical supplements. Int Immunopharmacol 2005; 5: 637– 47.
- 25, , , , , et al. The majority of in vitro macrophage activation exhibited by extracts of some immune enhancing botanicals is due to bacterial lipoproteins and lipopolysaccharides. Int Immunopharmacol 2008; 8: 1023– 32.
- 26, , , , . A novel melanin-like pigment derived from black tea leaves with immuno-stimulating activity. Food Res Int 2001; 34: 337– 43.
- 27, , , , , . Synthetic melanin suppresses production of pro-inflammatory cytokines. Cell Immunol 2000; 199: 25– 36.
- 28, . Alterations in the secretory pattern of dermal dendritic cells following melanin uptake. Cell Tissue Res 2013; 352: 599– 610.
- 29, , , , , et al. Aspergillus fumigatus conidial melanin modulates host cytokine response. Immunobiology 2009; 15: 915– 20.
- 30, , , , . Camellia sinensis tea melanin suppresses transformation of the aryl hydrocarbon receptor and prevents against dioxin-induced toxicity in mice. Int J Food Sci Technol 2008; 43: 261– 9.
- 31, , . Effect of melanin extract from black cumin seeds (Nigella sativa L.), on Humoral Antibody. Response to sheep red blood cells in albino rats. J Appl Anim Res 2006; 29: 337– 41.
- 32, , , . Immunostimulatory agents in botanicals, US patent 2005; 20050002962.
- 33, , , . Anti-inflammatory and immunomodulating properties of grape melanin. Inhibitory effects on paw edema and adjuvant induced disease. Arzneimittelforschung 1998; 48: 764– 71.
- 34, , . Toll-like receptor signaling and regulation of cytokine gene expression in the immune system. Biotechniques 2002; 8: 70, 72 passim.
- 35, , . A human homologue of the Drosophila Toll proteins signals activation of adaptive immunity. Nature 1997; 388: 394– 7.
- 36, , . Toll-like receptors. Annu Rev Immunol 2003; 21: 335– 76.
- 37, , , , , et al. Defective LPS signaling in C3H/HeJ and C57BL/10ScCr mice: mutations in Tlr4 gene. Science 1998; 282: 2085– 8.
- 38, , , , , et al. Differential roles of TLR2 and TLR4 in recognition of gramnegative and gram-positive bacterial cell wall components. Immunity 1999; 11: 443– 51.
- 39, , , , , et al. Endocytosed HSP60s use toll-like receptor 2 (TLR2) and TLR4 to activate the Toll/interleukin-1 receptor signalling pathway in innate immune cells. J Biol Chem 2001; 276: 31332– 9.
- 40, , , . Receptor-mediated monitoring of tissue well-being via detection of soluble heparan sulfate by Toll-like receptor 4. J Immunol 2002; 168: 5233– 9.
- 41, , , , , et al. The extra domain A of fibronectin activates Toll-like receptor 4. J Biol Chem 2001; 276: 10229– 33.
- 42, , , . Effect of herbal melanin on IL-8: a possible role of Toll-like receptor 4 (TLR4). Biochem Biophys Res Commun 2006; 344: 1200– 6.
- 43, , , , . Cancers take their Toll—the function and regulation of Toll-like receptors in cancer cells. Oncogene 2008; 27: 225– 33.
- 44, , , , , . Herbal melanin activates TLR4/NF-kappaB signaling pathway. Phytomedicine 2009; 16: 477– 84.
- 45, , , , . Ammonium hydroxide hydrolysis: a valuable support in the MALDI-TOF mass spectrometry analysis of Lipid A fatty acid distribution. J Lipid Res 2002; 43: 2188– 95.
- 46, . Effect of alkali on the immunological reactivity of lipopolysaccharide from Salmonella typhimurium. Infect Immun 1970; 2: 549– 55.
- 47, , , , . Quantification of bacterial lipopolysaccharides (endotoxin) by GC–MS determination of 3-hydroxy fattyacids. J Environ Monit 2004; 6: 65– 70.
- 48, , , . Biological properties of parent endotoxins and lipoid fractions, with a kinetic study of acid-hydrolyzed endotoxin. J Exp Med 1961; 114: 665– 84.
- 49, , , , , et al. Molecular requirements of endotoxin (ET) actions: changes in the immune adjuvant, TNF liberating and toxic properties of endotoxin during alkaline hydrolysis. Int J Immunopharmacol 1992; 14: 131– 42.
- 50, , , , , . What we know and don't know about the chemical and physical structure of lipopolysaccharide in relation to biological activity. Prog Clin Biol Res 1998; 397: 51– 72.
- 51. Molecules in focus: melanin. Int J Biochem Cell Biol 1997; 29: 1235– 9.
- 52, , , , , . Activation of microglia by human neuromelanin is NF-κB dependent and involves p38 mitogen activated protein kinase: implications for Parkinson's disease FASEB J 17:500–502. J Photochem Photobiol B Biol 2003; 63: 41– 51.
- 53, , , , . Following fungal melanin biosynthesis with solid-state NMR: biopolymer molecular structures and possible connections to cell-wall polysaccharides. Biochemistry 2008; 47: 4701– 10.
- 54, , , , , . Studies on biological activities of melanin from marine animals. inhibitory effect of SM II (low molecular weight melanoprotein from squid) on phenylbutazone-induced ulceration in gastric mucosa in rats, and its mechanism of action. Chem Pharm Bull 1985; 33: 2052– 60.
- 55, , , , , . Studies on biological activities of melanin from marine animals. Purification of melanin from Ommastrephes bartrami lesuel and its inhibitory activity on gastric juice secretion in rat. Chem Pharm Bull 1982; 30: 1381– 6.
- 56, , , , . Studies on biological activities of melanin from marine animals. Purification of melanin from Octopus vulgaris cunier and its inhibitory activity on gastric juice secretion in rats. Chem Pharm Bull 1982; 30: 1508– 12.
- 57, , , , , et al. Gastroprotective effect of an aqueous suspension of black cumin Nigella sativa on necrotizing agents-induced gastric injury in experimental animals. Saudi J Gastroenterol 2008; 14: 105– 6.
- 58, , , , , et al. Anti-ulcerogenic effects of Nigella sativa Melanin. 2009; Sudan Patent No. 1683.
- 59, , , . Anti-ulcerogenic Effects of Nigella sativa L. Melanin. World J Pharm Res 2016; 5: 1579– 93.
- 60, . Toll-like receptors and cancer. Nat Rev Cancer 2009; 9: 57– 63.
- 61, , , , , et al. Toll-like receptor-4 is required for intestinal response to epithelial injury and limiting bacterial translocation in a murine model of acute colitis. Am J Physiol Gastrointest Liver Physiol 2005; 288: G1055– 65.
- 62, , , , . Activated macrophages are an adaptive element of the colonic epithelial progenitor niche necessary for regenerative responses to injury. Proc Natl Acad Sci USA 2005; 102: 99– 104.
- 63, , , , . Recognition of commensal microflora by Toll-like receptors is required for intestinal homeostasis. Cell 2004; 118: 229– 41.
- 64, , . Toll-like receptor 2 enhances ZO-1-associated intestinal epithelial barrier integrity via protein kinase C. Gastroenterology 2004; 127: 224– 38.
- 65, , . Toll-like receptor 2 controls mucosal inflammation by regulating epithelial barrier function. Gastroenterology 2007; 132: 1359– 74.
- 66, , , , , et al. Toll-like receptors 2 and 4 are up-regulated during intestinal inflammation. Gastroenterology 2002; 122: 1987– 2000.
- 67. Toll-like receptor signalling in the intestinal epithelium: how bacterial recognition shapes intestinal function. Nat Rev Immunol 2010; 2: 131– 44.
- 68, . Differential alteration in intestinal epithelial cell expression of toll-like receptor 3 (TLR3) and TLR4 in inflammatory bowel disease. Infect Immun 2000; 68: 7010– 7.
- 69, . Role of Toll-like receptors in gastrointestinal malignancies. Oncogene 2008; 27: 234– 43.
- 70, , , , , et al. Toll-like receptor (TLR) 2 induced through TLR4 signaling initiated by Helicobacter pylori cooperatively amplifies iNOS induction in gastric epithelial cells. Am J Physiol Gastrointest Liver Physiol 2007; 56: G1004– 12.
- 71, , . The heat shock response and cytoprotection of the intestinal epithelium. Cell Stress Chaperones 2002; 7: 191– 9.
- 72, , , , , et al. Cox-2 is regulated by Toll-like receptor-4 (TLR4) signaling: role in proliferation and apoptosis in the intestine. Gastroenterology 2006; 131: 862– 77.
- 73, , . Regulation of colonic epithelial repair in mice by Toll-like receptors and hyaluronic acid. Gastroenterology 2009; 137: 2041– 51.
- 74. A feed-forward loop involving hyaluronic acid and toll-like receptor-4 as a treatment for colitis? Gastroenterology 2009; 137: 1889– 91.
- 75, , , , . The liver-protecting activity of melanin-like pigment derived from black tea. Food Res Int 2003; 36: 505– 11.
- 76, , , , . Protection of tea melanin on hydrazine-induced liver injury. Life Sci 2003; 72: 1061– 71.
- 77, , , , . Protective Effects of Tea Melanin against 2,3,7,8-Tetrachlorodibenzo-p-dioxin-Induced Toxicity: antioxidant Activity and Aryl Hydrocarbon Receptor Suppressive Effect. Biol Pharm Bull 2006; 29: 2284– 91.
- 78, , . Induction of cytochrome P4501A1 by 2,3,7,8-tetrachlorodibenzo-p-dioxin or indolo (3,2-b) carbazole is associated with oxidative DNA damage. Proc Natl Acad Sci U S A 1996; 93: 2322– 7.
- 79, , , . Protective action of herbal melanin against carbon tetrachloride induced Hepatotoxicity. Proc. of the Third Intl. Conf. on Advances in Applied Science and Environmental Engineering 2015; 1: 6.
- 80, , , , . Suppression of growth of cultured malignant cells by allomelanins, plant-produced melanins. Cancer Biother Radiopharm 1997; 12: 47– 9.
- 81, , , , , . Effect of allomelanin on tumor growth suppression in vivo and on the cell cycle phase. Cancer Biother Radiopharm 1997; 12: 273– 6.
- 82, , , , , et al. Dopamine-melanin induces apoptosis in PC12 cells; possible implications for the etiology of Parkinson's disease. Neurochem Inter 1997; 31: 206– 17.
- 83, , , , , et al. Effect of melanins from black yeast fungi on proliferation and differentiation of cultivated human keratinocytes and fibroblasts. Cell Biol Int 2003; 27: 135– 46.
- 84, , , , , et al. Studies on biological activities of melanin from marine animals. Anti-inflammatory activity of low-molecular-weight melanoprotein from Squid (Fr. SM II). Chem Pharm Bull 1987; 35: 1144– 50.
- 85, . Melanin: a scavenger in gingival inflammation. J Dent Res 2011; 22: 38– 43.
- 86, , . Anti-inflammatory effects of Nigella sativa L. Melanin. World J Pharm Res 2016; 5: 155– 61.
